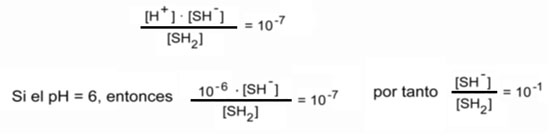1. INTRODUCTION
Sulfurations are chemical reactions that occur on the surface of some metallic containers with certain types of products which, by their nature, have a high thioprotein content:
- Cysteine: HS – CH2– CH(NH2) – COOH
- Methionine: CH3 – S – CH2- CH2- CH(NH2) – COOH
- Cystine: HOOC – CH(NH2) – CH2 – S – S -CH2- CH(NH2) – COOH
The cystine ↔ cysteine oxide-reductive couple depends on the redox potential of the product. The cysteine form is the preferred form in non-oxidizing, hence reducing, media (e.g. in the presence of the Sn/Sn++ couple).
In the second stage, the amino acids are decomposed into slphydryl compounds (mercaptans, SH2).
As far as SH2 is concerned, its dissociation into SH- and S-2 is pH-dependent:
This indicates that only one tenth of the SH2 is dissociated. It is also seen that the more acidic the pH, the lower the dissociation.
Similarly, SH- can dissociate into H+ and S-2 also under pH dependence.
It is necessary to retain this small chemical comment, that the phenomena of tin sulfuration are favored in low acid medium, and this is the case of fish, meats and certain vegetables (peas, cabbage, corn, etc…).
During a heating process, these thioproteins undergo decomposition, releasing sulfur ions, which can react with the metallic components of the container, such as iron and tin, giving dark-colored sulfides that spoil the appearance of the container and, in some cases, of the product.
In other cases, sulfuration may occur as a result of the use of certain sulfur compound additives, used in the first stages of the bleaching or preservation process, which have not been conveniently eliminated from the product to be packaged.
The appearance of sulfuration stains inside the containers does not cause any sanitary problem, since the sulfides formed are totally innocuous, and in many cases, they appear below the varnish, but they can cause an important commercial problem, since they affect the presentation of the product, especially if it has dark stains due to the remains of these sulfides.
2. SULPHURS
Inside the container, there are two materials, which, although in design are layered, it is possible for both to coexist together, in case of any mechanical damage on the surface of the material. These elements are tin, which forms the surface layer of the tinplate, and iron, a fundamental component of the base steel, which forms the tinplate.
Tin sulfide has a dark brown color and usually appears adhered to the surface, while iron sulfide has a deep black color and tends to detach easily, tending to stain the product.
Sulfurization on tin / Iron sulfurization
The electrolytic application of tin on the steel surface, in theory, should not leave iron exposed, but in spite of that, there is a certain porosity in the industrial electrolytic application, (higher the lower the amount of tin) that although it does not cause problems in acid packaging, since tin electrolytically protects the iron, it could cause microscopic spots of iron sulfide formation, which are usually not visible, within a dark surface stain of tin sulfide, much more extensive.
3. THE VARNISHES
The varnishes, applied on the tinplate, present a barrier effect to the attacks of the packaged product on the tin surface. This was supposed to solve the problem, but they continue to appear for two different reasons: On the one hand, a complete continuity of the applied varnish film cannot be guaranteed (pores, mechanical damage, etc.) and on the other hand, polymeric films, present permeability to gases, so, any sulfuration attack carried out by this means can occur, despite the use of varnishes, although in these cases, the sulfuration stains appear below the varnish, without contact with the packaged product.
In view of this, the permeability of the different coating applications must be assessed, and above all, the influence of the thickness of the film applied, but this factor is a compromise between its permeability and its flexibility to adapt to the mechanical requirements of the manufactured elements and of course the cost of the coating.
To try to avoid sulfidation, two possible solutions applied directly to the coatings have been presented:
- Use of pigmented varnishes, which mask the appearance of stains on the tin surface, as they are opaque varnishes (aluminum, white, etc.).
Use of chemically active varnishes, which neutralize the sulfur attack, avoiding the appearance of stains on the metal (addition of zinc salts). In these varnishes, the zinc oxide decomposes before the sulfur attack, forming zinc sulfide, also white, so the appearance of the varnish does not change after neutralizing the sulfuration. The problem that can occur is that the sulfidation attack is very intense, exceeding the amount of zinc contained in the varnish, in which case, dark sulfidation stains appear underneath the varnish, which are highlighted by the “milky” tone of the varnish.
4. THE PACKAGING MATERIAL
Tinplate also has an influence on the appearance of sulfidation stains. The tin surface in all cases is the same, and the formation of tin sulfide does not depend in any case on the amount of tin deposited on the steel. However, in addition to tin, tinplate receives a passivation surface treatment, in which chromium (and chromium oxides) are deposited as an antioxidant and hardening treatment. This deposited chromium is not susceptible to attack by sulfidation, so it is reasonable to think that the greater the chromium deposition, the lesser the problem. This is true, but the solution is not so simple: The higher the amount of chromium, the lower the adhesion of the varnish, and therefore problems can arise due to varnish detachment during machining.
There is an alternative, which is the use of a material that does not contain tin, the TFS (or ECCS), known as Tin Free Steel or chrome plate, which, having chromium deposited superficially, as we mentioned before, is not susceptible to sulfurization, but due to its chemical characteristics, it must always be used varnished.
There are two problems with this type of material, which means that it cannot be used in all cases, nor with all varnishes, and that it is currently in the process of disappearing due to legislative issues:
- On the one hand, today there is no technology that allows us to weld this material, so its use is reduced to the manufacture of lids and stuffed containers.
- On the other hand, due to the rigidity of the chrome film, it is necessary to use very flexible coatings, avoiding as much as possible the use of pigmented coatings (more brittle due to the solid particles they contain), so it is not recommended to use aluminum or zinc carbonate coatings, without the use of a flexible coating layer, under or on top of the main coating. The breakage of the varnish film exposes the chromium layer, which partially covers the steel, so that, in this case, the sulfuration formed is of iron, which, as mentioned above, is more intense and black, and susceptible to stain the product.
- Finally, we must consider its legislative problems, due to the possibility of Chromium (VI) content, catalogued as toxic, so that, in a short term, it could be prohibited by legislation.
5. THE PRODUCT
As is logical to think, the product to be packaged also has an important influence on the appearance or not of sulfuration stains.
When we talk about fish and shellfish, natural products (in water and salt) present a higher sulfuration, because in principle two factors intervene against them: on the one hand, a lower product viscosity, and therefore higher gas mobility, and on the other hand, and more importantly, a higher heat penetration, and therefore higher temperature, which causes a higher decomposition of thioproteins (at the same sterilization times).
Factors such as the variety, size or diet of the fish, and in some cases the bleeding, intervene more or less randomly in the formation of sulfurations, and these factors can be reduced with a scalding process prior to packaging, more or less long, that decomposes the highest possible degree of thioproteins, which logically would not reach the inside of the package.
With meats, something similar happens. Depending on the type of meat, as well as the different parts of the animal used, and the scalding or preparation process carried out, the sulfuration attacks will be more or less intense.
Finally, in vegetables, not only the variety of the product is involved, but factors such as the composition of the soil or water (higher or lower sulfate content) influence a higher or lower content of thioproteins, and as in the previous cases, a controlled blanching process can considerably reduce the occurrence of sulfuration problems.
6. THE PROCESS
An important part of the appearance of sulfuration stains in containers is the process to which the product and the finished container are subjected.
Firstly, thioproteins, as mentioned at the beginning, are decomposed by heat, giving rise to gaseous sulfur derivatives. In many products, prior to packaging, a previous blanching or boiling is carried out. In this process, part of the thioproteins are eliminated. If this process is more complete, as long as the texture of the product is not deteriorated, the decomposition of thioproteins is greater and, therefore, the sulfuration in the package will be lower.
The sterilization process of the container, together with its subsequent cooling, also has a decisive influence on the appearance of sulfuration stains. Thus, sterilization processes with long times and low temperatures are more sulfuring than short sterilization processes and high temperatures. In addition, in cooling processes, long cooling times result in much more intense sulfidation processes.
Finally, as the sulfur derivatives from the decomposition of thioproteins are gaseous compounds, sulfurations will preferably occur in the headspace, unless the product is highly viscous, making it difficult for the product and gases to circulate inside the container.
7. POSSIBLE SOLUTIONS
Given all these factors, it is easy to understand the difficulties that can arise to avoid the appearance of sulfidation problems inside the containers.
The best containers may be those made of materials that are not susceptible to sulfuration, such as TFS, but it is necessary to take into account what has been said about this material, considering the use of non-pigmented, highly flexible varnishes, to avoid breakage in the conformation of the containers and lids, which could lead to the appearance of black iron sulfuration.
But this is not always possible, since sometimes the markets are not accustomed to this type of material, and the “marketing” factor is also important for the decision of the type of packaging and varnish to be used, so that, if the packaging needs to be tinplate, the most practical is the use of opaque pigmented varnishes, which totally mask the signs of sulfuration. Thus, the “aluminum” varnishes, gray and white, are the most suitable for this type of problem, taking into account that the applications must be appropriate to the type of container and mechanical conformation, and that the handling of containers and lids with these varnishes is extremely delicate, since they can be easily scratched by the dragging of the solid particles that compose them.
Gold epoxyphenolic coatings, or gold organosol coatings, are permeable to a greater or lesser degree to gaseous sulfur compounds, and sulfidation problems may appear to a greater or lesser degree, depending on the various factors mentioned above.
Finally, the use of “active” coatings based on zinc oxide or zinc carbonate, as we have already mentioned, are effective up to a certain point, since they remain active as long as there is zinc compound left to neutralize the sulfurations, but once all the zinc has been consumed, the product is no longer effective. In addition, in any case, do not accept the presence of acid media, since the zinc salt is attacked, decomposing the varnish immediately.