The internal corrosion in tin can during storage, has its origin in the interaction between the can and the content. This can lead to two phenomena: the dissolution or migration of tin in the product or the same effect with iron. Although in certain cases, both occur simultaneously. The causes and means to prevent corrosion are often complex and can baffle even the most expert.
On the contrary, external corrosion results from the interaction between the container and the environment. The environment is made up of the atmosphere with its natural (oxygen, moisture) and non-natural components (gaseous residues, dust, sea spray, etc.). The result of this interaction, can appear quickly, in a couple of days and even in a few hours and manifests in the form of rust spots.
Although the contents of the container are not endangered by this type of corrosion, it must be admitted that the sale of the same at retail and wholesale can be seriously compromised. In the same way that people are judged by their appearance, sometimes unfairly, a rusty container will not inspire confidence and is associated with the idea that they have surpassed their lifespan on the shelves. The contrast with contiguous containers “healthy”, will increase this bad impression.
The purpose of this article is to show that the resistance to corrosion of tinplate or TFS is not an absolute phenomenon and is strongly related to the conditions of use of the material. We will focus particularly on the latter. We think that some simple precautions are enough to avoid the oxidation (sting or stain) of all these materials.
We begin by saying that it is the porosity of the tin coating (or chromium), which affects the conditions necessary for the final formation of the oxide. A sufficiently thick “thick” tin coating – preserving packaging from scratches and abrasions during the production process, its canning and travel along the commercial circuit – would undoubtedly constitute the best barrier against rust formation. However, low-coated tinplate (for example less than 2.8 g / m2) is technically and economically justifiable.
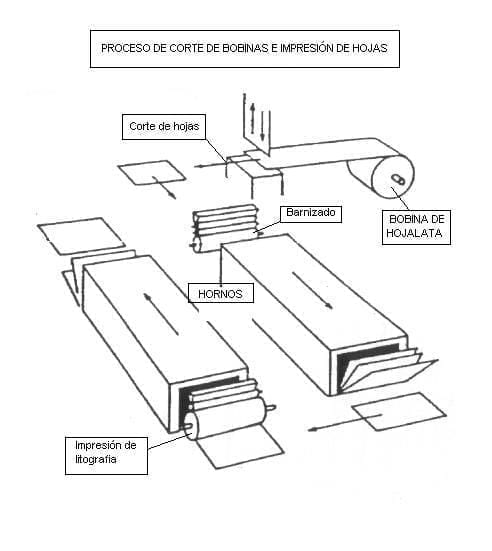
It is well known that tinplate is composed of a series of layers of very different thicknesses, which are basically:
a.- A steel core, which forms the basis of it, providing it with its mechanical properties.
b.- Wrapped to it – and for its protection – tin is used, which is used for its chemical inertia in relation to the resistance to internal and external corrosion.
c.- A layer of tin / iron alloy is located in the “interface” of the two aforementioned layers, which originates during the surface treatment of deposited tin. This layer also has a relevant role in corrosion resistance.
d.- Passivation, very thin layer applied on the outside of the surface of the tin, provides the material, depending on its type, specific surface properties that affect the sulphuration, adhesion to varnishes, etc.
e.- The oil layer, normally of nanomolecular thickness, should reduce the damage caused by abrasion during any manipulation of the tinplate.
When studying the corrosion resistance, we will consider only the components a and b, iron and tin:
– steel, because it generates oxide (iron oxide)
– Tin, because the degree of protection of steel is related to the thickness of the tin coating.
The other tin components in this aspect have very little or no impact.
Faced with the study of corrosion in tin containers, three essential questions are posed:
-What are the inherent factors of corrosion?
-What are the corrosion factors external to the material and the container?
– Are there preventive measures for corrosion?
Why can tin oxidize?
Considering the first question, consider the material delivered by the tin train.
In all the low thickness coatings, the tin coating is not continuous, there is always a certain degree of porosity. In the tinning train, the porosity decreases (exponentially) when the tin coating mass increases and becomes non-existent only when the coating exceeds 50 g / m2. This value is much higher than the highest coating masses ever applied in the past on hot tinplate (“coke” tinplate).
It is easy to show the correlation between the rate of oxide formation and the thickness of the tinplate or chromium coating (in the case of TFS).
Accelerated oxidation tests are performed on samples taken from the tinning train. For example, they are alternatively subjected to condensation and drying cycles in a hot, humid saline atmosphere.
At the laboratory level, where the conditions of the tests are perfectly controlled and comparable, when averages are taken after a certain number of tests, statistics show that the coating tin E 5.6 g / m2 has a better resistance than the E 2.8. However, it may happen that individual 2.8-sheet tinplate samples show a higher corrosion resistance than some 5.6
Regarding the surface finishes of the metal (shiny, matt or stone) it can not be said that different resistance values are obtained depending on them.
The mechanical stresses, scratches and abrasions of all kinds supported by the material during the manufacture of the container and its filling and processing in the canning factory, will greatly increase the amount of the exposed surface of the steel, particularly in the areas of the seams, seam lateral, cordoned areas, etc. The original porosity that exists in tinplate is quite insignificant when compared to the porosity resulting from some areas of the finished and full container.
Tinplate being a material that is prone to oxidizing in areas where the tin coating has been damaged, it is evident that the treatment that the container receives throughout its useful life has a decisive influence: filling, storage, transport …. The conditions in which these operations are carried out can make a difference.
– If stored in good condition, any tin, full or empty container, even with a low tin coating, may be safe from oxidation.
– If stored in poor condition, any tinplate, full or empty, even with a high coating, can oxidize quickly.
There are “bad conditions” every time the steel base of the tinplate comes into contact with an electrolytic medium prone to become a corrosion cell. The aggression of the medium varies with the nature of the dissolved salts and increases considerably with temperature.
What are the oxidation factors, external to the material and to the container ?
The presence of a certain number of dangerous conditions favors the appearance of rust spots. Mainly during:
A-The process in the canning factory
B-The storage, shipment and sale of both filled and empty containers.
A.- The conditions of the process in the canning factory
1.- Containers stained during the filling operation, for different reasons such as: filling too much, spilling liquids (syrups, brines), etc.
When the outer part of the container is exposed to the contact of acids (fruits) or saline substances (brines), these will act as an electrolyte of corrosion cells. These substances can also contaminate the process water, which evaporates on the containers, leaving a film of hygroscopic and corrosive products. In the same way, during the vaporization phase, if the temperature is high enough to cause foreign substances to dry out and stick strongly to the tinplate, hygroscopic areas with a strong propensity to corrode the metal can result. Mainly the areas where the tinplate has undergone the most stress will be affected, for example in the side seam, closures, laces and expansion panels.
In steam autoclaves, if the process takes too long to reach the right temperature, if there is previously an oxidation point and if the vapor contains too high a percentage of air, oxidation spots can form as a consequence of all this.
A boiler, when operating above its normal capacity, can supply steam that is loaded with alkaline substances, which will attack the tin coating of the container in the most exposed areas, even under a protective coating of varnish. This unpainted resulting from tinplate also generates an increased sensitivity to corrosion of the metal at the time of cooling and storage of the containers.
Throughout the sterilization process, the containers can become coated by whitish deposits (carbonates, sulfates, chlorides …) transported by the steam and are difficult to eliminate during cooling. Said salts are hygroscopic and therefore cause a subsequent corrosion and oxidation during the storage of the containers.
The steam loaded with the condensed alkaline substances, accumulated in the autoclave during the process, can attack the containers located in the lower part of the cages or containers.
The internal state of the autoclave cages (oxidized, newly galvanized and / or with separators in poor condition), can also be the source of corrosion (sometimes caused by eddy currents and any problem of “bimetallism” in the autoclave).
In the case of a “bain-marie” process, or in a static autoclave, the corrosion of tinplate, with the consequent formation of oxidation stains, can be caused by oxygen dissolved in water below the boiling point or by alkaline substances transported by water vapor drag.
In some continuous autoclaves, the containers are subject to a rotation, which if the penetration of the heat is greatly improved, can also increase the degree of abrasion due to friction.
2. Cooling and drying containers improperly
Corrosion may appear when cooling is excessive. In this case, the container is too cold to dry spontaneously and an excessive amount of water is retained, particularly in the areas of the seals and side seam. Regarding this, it is preferable to wash a hot container with hot water instead of washing or cleaning the cold containers with hot water. The ideal way, to ensure the most effective cleaning and drying, is that the containers still hot (approximately 40 degrees Celsius) should be washed and rinsed with hot water. IN NO EVENT shall the canner proceed to pack the containers in cardboard boxes before they are completely dry, especially if the cardboard hinders the rapid evaporation of moisture. The plastic wrapping of the containers arranged in trays is often the cause of rust spots due to the difficult evaporation of water droplets or residual moisture.
B.- Conditions of storage, shipment and sale of full or empty containers
The factors to consider are:
– Hot and humid atmospheres, full of dust, saline dew from the sea or gaseous residues.
– The “sweating” of packaging or condensation.
– Secondary packaging such as cartons or boxes (including adhesives and labels).
1.-Atmosphere
An excessive degree of humidity, together with a high atmospheric temperature during storage, are perhaps the most important causes of external corrosion of the container. Below 60% relative humidity, corrosion can be considered non-existent, while above 80% becomes important.
It is necessary to emphasize that the floors, walls and cartons can absorb enough atmospheric humidity to accelerate the corrosion of the containers. The accidental wetting of cardboard boxes in a canning factory, for example, can cause corrosion. The same phenomenon occurs with paper rolls wrapped in paper.
The aggression of the humid atmosphere in contact with the containers can be increased by the presence of dust and salts, as well as gaseous residues (sulfur dioxide, chlorine …)
2.- Condensation or “sweating”
The condensation of atmospheric humidity on the containers is also a very important cause of the external corrosion of the same. Condensation or “sweating” occurs when cold containers are suddenly exposed to air that is at a higher temperature and humidity. Spring is the most propitious period for condensation in the warehouse. Thus, when cold stores are open on a hot and humid day there is a greater risk of condensation of moisture on the containers.
The speed of condensation depends on:
– The temperature of the containers.
– The ambient air temperature.
– The relative humidity of the air
Under identical conditions, empty containers are less prone to condensation than those that are full, undoubtedly due to a faster equilibrium of temperatures
3.-Secondary packaging, cartons, adhesives, labels
The boxes and / or cartons can be the source of external corrosion of the container. Certain grades of cardboard absorb more moisture than others. The nature and quantities of residual salts in cartons (sulfates, chlorides …) affect the aggressiveness of cartons in relation to packaging. It is the closures that suffer mainly from corrosion, because the weight of the containers causes them to be “stuck” in the wet cardboard. The material used to build wooden boxes or pallets, can cause contact corrosion, when the humidity level is above 15% (freshly cut wood for example)
Certain adhesives and adhesives used to stick the labels are hygroscopic and therefore prone to cause oxidation stains on the containers. Those labeled with paper are exposed to corrosion, especially in the case of waterproof labels (glossy or plasticized).
In summary, we can say that, under all these conditions, we find three basic factors: humidity, oxygen and acidic or alkaline substances, which act together with temperature to play an aggravating role in relation to the appearance of rust.
C.-How to prevent pitting and corrosion spots on containers
Based on the information already indicated, we can give some practical advice:
We believe that a significant increase in the tin coating mass is not the best solution, both from an economic and technical point of view, if the risks of external corrosion of the container due to adverse conditions can be controlled and reduced. However, the use of varnishing and printing with inks is a very good solution. In general terms, it is worth emphasizing the following points:
– The storage of empty and full containers must take place in warehouses that are completely separate from the canning facilities. In the canning factory, excessive humidity prevails constantly due to the cleaning of the floors, the steam in the pots and autoclaves, while the acidic humidity is present in the air coming from the brine.
– Cardboard boxes, when damaged, can not be expected to protect containers from splashing water and spills. The same goes for paper rolls wrapped in paper.
– The containers should be washed and rinsed with hot water before placing them in the autoclaves. The detergents used for this type of cleaning must be neutral in relation to the containers.
– In the case that the containers are sterilized in water, if this water – treated its hardness – is recycled, it is important to check at regular intervals certain physical and chemical characteristics, to be able to control the variations that concern the hardness of the water , its pH, the content of chlorides, nitrates, etc.
– The cooling of the containers after their sterilization process should not be excessive. At 40 degrees Celsius, the containers still retain enough latent heat for spontaneous drying.
– The chlorination of cooling water to avoid recontamination of the containers after processing, should not be a factor that favors corrosion as long as the free active chlorine does not exceed 2 to 3 mg / liter at the time of use, ie at the time of contact with the containers that are cooling. Said content must be kept under control and checked at regular intervals.
– When palletizing or packaging the containers in cardboard boxes, the containers must be completely dry and free of white deposits that have originated from previous washes. A wet or insufficiently dry container is highly prone to corrosion, especially if the box material is not moisture proof. It is a common practice that the boxes should not be wet and that the hygroscopic material for the boxes should be rejected.
– It is recommended that special attention be paid to the shrink wrapping of flexible or plastic wrappers, which can be the origin of local rust spots due to the generation of numerous internal “microclimates”.
– There are corrosion inhibitors that can be added to the sterilization water or to the water in the packaging cooling equipment. These active agents are included in the commercial formulations and in this way their compositions are not always well known. It is not our intention to criticize the use of these products. On the contrary, we believe that in some special conditions, they are useful. We should note however that:
– Any product should not be used (they must be authorized since containers are involved to contain groceries).
– Its use creates complications in the supervision task in the canning factories and its verification is necessary. These products generally act in small quantities and there is an optimum concentration above which all effective action is risky or paradoxically, there is an increase in corrosion.
– The effectiveness of these products is not eternal. It is often limited to the medium in which they act. In this way, for example, they can prevent the water from being aggressive when it is in contact with the containers, but when they leave or leave this medium, they will be exposed again to new influences if there are poor drying and treatment conditions. It is one thing to make the water of the autoclave or cooling less aggressive, and another thing is to deposit a protective layer, by means of this water on the outer surface of the containers, capable of considerably retarding the risks of corrosion under adverse conditions of exposure.
– It is also possible to subject the filled and dried containers to a vapor of a hydrophobic substance (oil, paraffin, etc.). But what will happen, however, in the labeling process? There may be problems with the adhesives.
The above comments show that the use of corrosion inhibitors is not always clear and for this reason they should be used only as a last resort, after all other established precautions have been fully met.
A dry, cooled container, stored in an air-conditioned warehouse, protected from dust and especially condensation, can by itself resist atmospheric corrosion for a long time. Moisture condensation in full containers can occur with reduced temperature differences between ambient air and containers:
– Approximately 3 degrees Celsius, when the temperature of the containers is below or equal to 10 ºC.
– Approximately 5 degrees, when its temperature is above 10 ºC.
To avoid the risks of condensation, it is always important to use warehouses in which humidity and temperature variations can be kept under control at the different points of the warehouse (using air conditioning, insulation, heating systems that provide heat and humidity-free air). ).
When corrosion incidents of containers are detected, it is essential to thoroughly investigate all possible “on-site” canning factors. It is equally important to check if there has been a failure in the precautions at a specific time.
In our opinion, the implementation of a reception test, to determine the oxidation resistance of the tinplate, at the time of delivery by the manufacturer of the material, is utopian, precisely because of the diversity of situations. In the end all this depends on the conditions of use of the containers.













can i ask for a permission for this copy to be printed