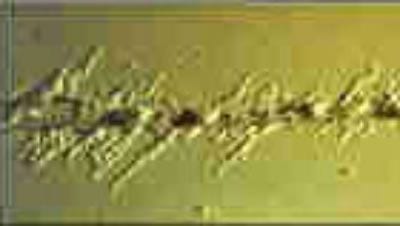
Filiform corrosion is a form of attack in which the corrosion process manifests itself in filaments and represents a particular type of anodic undercut. It is usually produced in humid environments and is more common under organic films applied on steel, aluminum, magnesium and zinc (galvanized steel). Sometimes, it develops in bare steels on which small amounts of contaminating salts have been accidentally deposited. It has also been observed in thin electrodeposits of tin, silver or gold. The filaments formed by the corrosion products show a wide variety of configurations from the nodular (aluminum case) to the very fine and well defined observed under the transparent lacquers applied on steel. The width of the filaments ranges from 0.05 to 0.5 mm and, under laboratory conditions, they can grow at a virtually constant speed (between 0.01 and 1 mm) for a long time. For its development, the filiform corrosion requires a relatively high humidity,> 55% at room temperature; Its speed can be accelerated by making a cut in the film that reaches the metal substrate and then maintaining the sample at a minimum relative humidity of 70 to 85%. Certain properties of the film, such as adhesion to the metal surface, also have an effect on the extent and character of the attack.
Up to the present there is no concrete explanation but it seems that the limited availability of oxygen and water that diffuse through the coating is one of the determining factors. At very high relative humidity or in contact with liquid water this type of corrosion stops quickly to a more general one, losing the filamentous character.
The filiform corrosion in tinplate takes place under protective films, and is usually called corrosion under film. It is a form of corrosion in cracks. Unlike other types of corrosion, it does not seem to weaken the component but it affects the external appearance. This is of particular importance in the metal packaging industry.
Currently, the reasons for filiform corrosion in tinplate are not clear, and since this is a growing problem, more work must be done to control the causes and effects
To deal with the causes of filiform corrosion, two aspects must be taken into account:
1) Base material.
2) Varnish.
1) Base material
1) On a steel base without varnish, filiform corrosion should not occur. This is because it is believed that the original cause includes the use of some type of film with oxygen below the varnish. The film is in most cases a semipermeable membrane, and allows the exchange of air with the atmosphere.
However, filiform corrosion has also been observed in baked TFS material. The complete mechanism is not known, but is believed to be due to the fact that baking causes the hydrated chromium oxide to become insoluble, thus forming a permeable membrane, which can be classified as a film under which the corrosion.
2) Low-tin-coated materials when there is any exposure to steel (usually due to the electrolytic tin-plating layer) are susceptible to filiform corrosion.
3) High weights of the oil film, which reduce the adhesion of the varnish, can cause air pockets, essentially in flexible varnishes. These can then act as propagation factors of the filiform corrosion.
4) The presence of anions, such as chlorides and bromides, and ligands of the chelating agents of the varnish, such as polyamides in the material, before varnishing, can also act as initiators of this type of corrosion.
5) In many cases, where the cutting edge of a component is exposed to an atmosphere or aggressive environment, corrosion may start at the edge and then proceed below the film. This form of corrosion moves in line, resembling the roots of a tree.
2) Varnish
1) Poor curing of a varnish can allow the residual solvents, polymers or ionic species generated by the curing process to remain below varnishing. It is known that low baking is an important cause of filiform corrosion.
2) Excessive curing, where the varnish becomes brittle also seems to make the components susceptible to filiform corrosion
The coating cycle on the tinplate also produces an increase in the alloy layer exposing the iron ions.













0 Comments