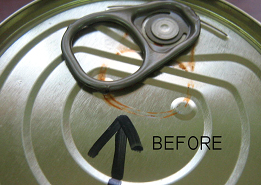
In some cases, the industrial process of canning with metallic steel containers can cause oxidation phenomena on both the container body and the lids, causing commercial rejection of the containers.
These rust stains, which are caused by leaving wet containers after sterilization, can be eliminated by chemical treatment of the containers, which produces a process consisting of dissolving the rust formed and subsequently passivating the unprotected areas to prevent the formation of new corrosion in warehouses. It has been proven on many occasions that the treatment described is effective for removing rust stains caused by boiler dragging, and for oxidations that are not very deep or have formed below the varnish film.
The chemical treatment consists of dissolving orthophosphoric acid (H3PO4) in water. The acid concentration should always be determined by means of initial treatment tests, trying different concentrations and times depending on the type of oxidations and the external varnishes on the container and other elements of the container, such as easy-open lids and rings, to achieve an effective process to eliminate rust without affecting the varnishes on the containers and lids or their components.
According to our experience, the concentration of pure acid in the treatment solution ranges from 3 to 5 %. Higher quantities increase the risk of attack on varnishes and easily opened rings, and do not improve the effectiveness of cleaning. It should be noted that the most effective treatment consists of an adjusted concentration/treatment time process, and the adjustment of each of these parameters should be set on a case-by-case basis.
Two factors must be taken into account in order to carry out the treatment:
⦁ The treatment temperature should be kept constant at about 75-80 ºC throughout the process.
⦁ The treatment lasts between 2 and 4 minutes, depending on the concentration and the containers, and if possible shaking the containers or the solution every minute, so that the attack of the product is more effective.
Once the containers have been processed, in solution, they should be washed immediately with clean, hot water, which should be changed regularly to avoid raising the acid concentration in the solution. The reason for using hot water is to promote the self-drying of the containers, once the washing process is finished.
The duration of this washing process can be from 1 to 3 minutes, depending on the size of the container, in order to achieve the superficial heating of the containers, which subsequently causes the self-drying of the containers, without affecting the quality of the packaged product.
Care must be taken to ensure that the containers are completely dry after treatment (hence the use of hot water during washing), in order to avoid subsequent oxidation phenomena. Only if the containers remain cold and wet can new corrosion processes occur.

This process can be carried out, for example, in open sterilization cauldrons, one of which can be used for treatment and the other for washing. These cookers should be made of metal, preferably stainless steel, to avoid acid attack. In case they are made of iron, they should be initially treated with an antioxidant treatment to avoid the contribution of oxides to the water, which could cause problems of galvanic batteries and, if necessary, the absorption by the repair varnish of the waterproofing cover.
Finally, it must be taken into account that those areas where the rust is removed, or are unprotected (steel in the air) will be dark gray, due to the process of phosphatization of the steel that this process causes, and that in part protects from subsequent oxidation.
This process is not applicable for oxidations produced by prolonged storage in unfavorable conditions, such as storage with high humidity, temperature changes and dew point condensation, contact with humid or high salt content cartons, which produce filiform or heavily encrusted corrosion.












0 Comments