SUMMARY
Description of several industrial procedures for the recovery of tin contained in tinplate scraps discarded by the metal industry. From them it is deduced that the really interesting one is the one that obtains this tin by a dry process by leaching with chlorine.
INTRODUCTION
Tin is the most specific component of tinplate, which gives it its main characteristic: its resistance to corrosion due to the action of external elements. It is a metal of high price in the market, since its obtaining from ore, besides being expensive, is very controlled by large multinational companies that try to maintain the high price and shortage of supply before an important demand. Therefore, in certain periods and in some geographical areas, it is often interesting to consider the recovery of this metal from discarded tinplate cuttings from the packaging factories. That is why we consider it interesting to partially reproduce an article by Pedro López Gómez published under the title “THE RECOVERY OF TIN TIN” in the magazine “Técnica Industrial”
“The tin content in the tinplate is variable, between 2% in the thick plates to 4.5% in the thin plate. As a general criterion, cuts that do not contain at least 2% tin can not economically benefit.
It must be extended to the unpainted so that the Matin steel support – tin core – can be used again as scrap. The presence of tin, even in small quantities, exerts a considerable influence on the ductility of the steel and a content of 0.3% of Sn is enough to make it brittle and inadequate as recovery steel.
As we all know tinplate is formed by an outer layer of almost pure tin, easily separable; Below is another crystalline layer formed by a real tin-iron alloy that is more difficult to separate because it is strongly adhered to the support plate and can be difficult to attack.
PRELIMINARY WORK
Before applying any method of destaining it is necessary to thoroughly clean the tinplate cuttings and eliminate as much fats, inks, varnishes and organic residues as possible.
To do this, perforated packages are prepared that undergo a kind of preliminary fusion at low temperature to eliminate as much tin as possible. Prior to this operation, the varnishes covered by the cuts must be eliminated. For this, the varnish dissolves easily in an alkaline lye of Solvay soda or 10% caustic; if enameled residues are observed, the tin packets are passed through a calender of rollers arranged so that one of the cylinders works at a higher speed than the other, with which the pack undulates and the enamel leaps.
There are equipment on the market such as “Goldschmidt TH”, which presses and punches the cuttings with barbed cylinders, and saponifies fats and varnishes with a treatment with sodium hydroxide at 3% for fifteen-thirty minutes in an autoclave at 110- 115 º C, then washed with hot water to remove soaps and bleach and then heated for half an hour at about 500 º C to recover the outer layer of tin and thus destroys the organic matter – closing gums – and re – pressed.
In this operation he has taken great care not to form a cock of organic matter, which would later hinder the penetration of the destaining agents.
The known processes of destaining can be classified into four large groups:
a) .- Chemical processes by wet process.
b) .- Dry metallurgical processes
c) .- Electrolytic processes by solution in acids or alkalis.
d) .- Processes that use dry chlorine
We will see each of them below.
CHEMICAL PROCESSES VIA THE HUMID ROUTE
Tin tin has been recovered for a long time using chemical processes of solutions of acids, alkalis or saline solutions, desisting from continuing its use for having low yields at all times.
Kecth and Hette started this path with an eye on a subsequent electrolysis; but Goldsch-Midt showed that dissolving tin by acid dissolves at the same time iron, of difficult and costly separation. Alkalis alone or added with oxidizing agents do not attack iron, nor do they completely eliminate tin, so that the steel in the sheet can not be used industrially either.
Schulze treats tinplate with stannic chloride solution; This dissolves metallic tin to be reduced to stannous salt:
Cl4Sn + Sn = 2 Cl2Sn
And then water vapor passes through, which precipitates stannous oxychloride and regenerates the stannic chloride, which returns to the cycle:
6 Cl2Sn + 2 H2O + O2 = 4 ClSnOH + 2 Cl4Sn
The separated ClSnOH is filtered and transformed into a SnO 2 stannic oxide by oxidation to red in the presence of air with chlorine evolution, which is recovered:
4 SnClOH + 3 O2 = 4 SnO2 + C l2 + 2 H2O
Ferric chloride was also used instead of stannic chloride as a solvent, because if Cl 3 Fe is present in excess, the tin is oxidized and the iron is reduced:
4 Cl3Fe + Sn = Cl4Sn + 4 Cl2Fe
When almost all the ferric chloride has oxidized the tin forming stannic chloride, it is reduced with the tin in excess present and passes to stannous chloride.
Cl4Sn + Sn = 2 Cl2Sn
That with water vapor is transformed into stannous basic chloride and subsequently calcined obtain stannic oxide and recover the necessary chlorine to regenerate ferric chloride by oxidation of Cl 2 Fe:
2 Cl2Fe + Cl2 = 2 Cl3Fe
That goes back to the leaching cycle; in both cases the stannic oxide is reduced to metal in a suitable furnace.
Reinecken-Ponsgenykopp treats the tin waste in rotating drums with PbO – flea rust – and water vapor, thereby achieving – in the presence of the previously added soda lye – to form sodium stannate SnO 3 Na 2. 3H 2 O and separate metal lead.
The sodium stannate can be placed on the market, concentrated or crystallized, or in a subsequent operation, precipitate the tin by a gaseous stream of carbon dioxide, and subsequent reduction in a reverberatory furnace.
METALURGICAL PROCESSES BY DRY ROAD
This process is reduced to a separation of tin by melting and oxidation in part to “tin ashes”, which are reduced in a reverberatory furnace or dissolve in molten metals, or to directly form salts or a use of the metal by simple fusion and mechanical separation of liquid tin drops.
Laroque, for example, mixes the tin waste with charcoal – which has hardly any ash – and sodium chloride, and Edmunds melts the tin snips at sufficient temperature to liquefy the tin in a centrifuge provided in its central part of a special hearth, with which the molten tin droplets are separated by centrifugal force.
Wolterez treats tinplate residues in retorts with water vapor reheated at 450-500º C, to separate tin from iron by melting; in these conditions, tin does not oxidize because it is exposed to the reducing action of hydrogen produced by reduction of water vapor over reheated iron; only a faint rust film can be detected on the outer layer of the liquid tin; processes that cause molten sodium to act on the tinplate cutout have also been tested; they have the advantage of completely dissolving the tin, with what remains a cut of steel in unbeatable conditions so that with a simple slab it can be used again, but the process is complicated, annoying and dangerous, as can be deduced from the progress of the process great traits we relate: a series of watertight deposits and conveniently linked with pipes, all this was built in steel and worked as a exhaustor; in the header, the tinplate cuttings are introduced together with pure sodium or sodium-tin alloy; once the appliance was hermetically closed, it reheated at 400-500ºC in a gas oven; the contents were stirred and the content was stirred, so that the tin dissolves in about fifteen minutes: continuing leaching in a continuous cycle leads to a 50/50 alloy; now it only remains to recover the sodium by distillation to obtain the tin.
ELECTROLYTIC PROCESSES
At first we worked with acid electrolyte: sulfuric acid, sodium acid sulfate and chlorides; With this type of electrolyte the yields were low and it was soon abandoned. Today the basic electrolyte is used exclusively, but we are going to give a slight idea of how it worked in its time.
Gutensonn worked with a 10% sulfuric acid electrolyte heated to 60º C. It formed the anode with the tin packets placed in wooden baskets of 1200 x 300 x 800 mm. that is to say, with a volume of 0.288 cubic meters, while the baths of approximately one cubic meter of capacity were constructed with pine or beech wood 50 mm thick, covered with 3.5 mm thickness of compressed rubber. The cathodes were formed with tinned copper plates of 1200 x 0.50 x 1.5 mm; in each bath there were eight anodic boxes and 16 cathodic boxes 100 mm apart from each other. The working characteristics were 240 A, 15 V that theoretically should separate 4.15 kg. of tin per hour, but the practical yield barely reached 45% for spending the rest of the current in works of dissolution of iron and evolution of hydrogen, so that a fairly pure tin was obtained.
In the alkaline process the electrolyte is 10% NaOH, the rest remains; now the tin passes to dissolution as sodium stannate of SnO 3 Na 2 formula, very soluble that precipitates in metal form on the cathode.
The precipitation is slower than the dissolution, with which the electrolyte is gradually enriched in tin, although with decreasing speed.
A particular enemy of the current performance is the avidity of the sodium lye of the electrolyte by the atmospheric carbonic anhydride; When carbonated NaOH loses dissolution activity and electrical conductivity, which imposes the easement of renewing the electrolyte too often to by causticization remove the sodium carbonate formed and impurities dissolved at the anode; It is convenient to remove the electrolyte frequently so that the anode only yields stannous ions.
The working temperature is centered in the vicinity of 70º C with an anodic density of 18 A / m2 and 100 A / m2 in the cathodic region, so that if the electrolyte contains 10% free alkali, the current yield Anodic is located in 94-95%, if the electrolysis suddenly stops, the stannous ions disappear and an elevation of the terminal voltage of 0.7-1.2 V is manifested immediately.
Now, in all likelihood, the iron-tin alloy begins to unravel; the voltage increases rapidly to 1.8 V and only 0.08% of tin remains in the sheet; that is, approximately 2.40% of the total amount to be recovered; Current performance is now 88-89%, which decreases to 82-83% as the voltage increases to 2.5 V and only 0.02% of Sn remains to be recovered, which represents 0.05-0.06% of the amount contained primarily in the clipping
The retraction of electrical performance shows that the anodic work is mainly focused on iron; This becomes passive and releases oxygen, which lowers the current performance.
The color of the anodic residue is not determinant nor indicative of the degree of tin leaching; if with a content of 0.28% Sn it is bluish gray and with 0.02% reddish Sn, and with 0.08% it shows reddish efflorescence.
On the other hand, if the electrolyte is totally or partially carbonated or the agitation is not very vigorous, the degree of destaining decreases little, but the terminal voltage increases and the current yield decreases.
The percentage of tin in the electrolyte should not be higher than 2.34%; otherwise, saturation makes anodic attack difficult, and as an unpleasant sequence, unsaturable hydrolyzed, non-recoverable, stannic acid can be formed which is lost in anodic slimes.
The alkaline process faster and cheaper than acid requires a lot of vigilance; on the method to the acid electrolyte presents the advantage that produces a steel residue of great acceptance in the steelworks, while the anodic residue of the process that works with acid electrolyte can only be used to obtain ferrous sulfate.
The acid process has, on the other hand, the advantage that tin passes to the solution as a stannous ion, with which, theoretically, with the same amount of coulombs, a double amount of tin metal must be separated.
DRY PROCESS BY LEACHING BY CHLORINE
The word leaching comes from the Latin: “Lixivia, -ae” feminine noun meaning bleach. The Romans used this term to refer to the juices that the grapes exude before stepping on them, or the olives before grinding them. At present, it is called leaching, when washing a pulverized substance to extract the soluble parts.
Proposed by Higgins and perfected by Parmelee, Lambotte Goldsmitd and Weber, it has industrially swept the other extraction methods because dry chlorine easily dissolves tin and allows it to be separated in the form of stannic chloride without attacking the support steel; Germany recovers over this process more than 85% of the production of cuts.
Winteler obtains stannic chloride by making dry chlorine on the tinplate in cuts at a controlled temperature of 40-50 °, since in this temperature range the chlorine does not attack the iron; for this to happen it is necessary that the temperature in the leach reactor exceeds 100 ° C; if ferric chloride is formed by carelessness, it will be said in metallurgical terms that the “can has burned”; it must be taken into consideration that the reaction:
2Cl2 + Sn = Cl4Sn
Releases 127.25 Kcal / mol. of stannic chloride formed, which can nullify the leaching performance by raising the temperature that occurs. The critical point focuses on the entry of chlorine into the reactor; Due to the high specific density of the chlorine atoms present, which are eager to react, a light layer of anhydrous, crystalline ferric chloride can form which, due to its hygroscopicity, absorbs moisture from the enclosure and from this moment dissolves the iron in the form of trichloride. iron can manifest with tremendous speed in the whole sheet; This problem was solved by Weber J. intensively cooling the reactor, which improved the process and obtained hot water for washing the recovered can.
Tinplate tin recovery technique focuses on the property of dry chlorine to easily attack tin and respect the support iron as long as there is no trace of moisture in the reactor; economically it can not be viable if sufficient quantities of cheap dry chlorine are not available and with this process in a single operation the two components of tinplate are obtained simultaneously. On the one hand, stannic chloride of great value for its application in the dyeing of textile fibers – silk, etc. -, and on the other hand, steel or sweet iron perfectly unstained with less than 0.1% Sn. In contrast to the other methods of recovery, the process of destaining by dry chlorine only has advantages:
– The first and second processes presented are out of date, with uncertain results, require expensive facilities and the cost of recovered tin does not compensate more than under special conditions of a country.
– When it is electrolyzed with the acid electrolyte, too much energy is consumed, since 50-35% of the total quantity of faradays supplied is consumed in works of dissolution of iron and generation of hydrogen; the facilities are onerous, very exposed to suffer continuous and serious breakdowns as all those that work with acidic liquids, with which the economical yield is not brilliant and the tin is too expensive to be put on the market.
– The electrolysis of basic electrolyte presents some technical advantages over those of acid electrolyte; However, its high energy consumption – 3,000 kWh / Tm – of recovered tin does not support the treatment at the price at which the kWh is invoiced, and because it needs too complex installation, as things are today, it would be a miracle miracle that could give Economic performance.
– Unpairing by the action of dry chlorine on the tin cuttings solves the problem elegantly and simply; it needs little installed force, little personnel, little monetary immobilization and the by-product can be sold well to the steelworks without problems after a simple washing.
Liquid chlorine is found without much difficulty – it is manufactured by Solvay and Cia. – The most difficult problem is to establish a consistent tinplate cut-out collection line that works well.
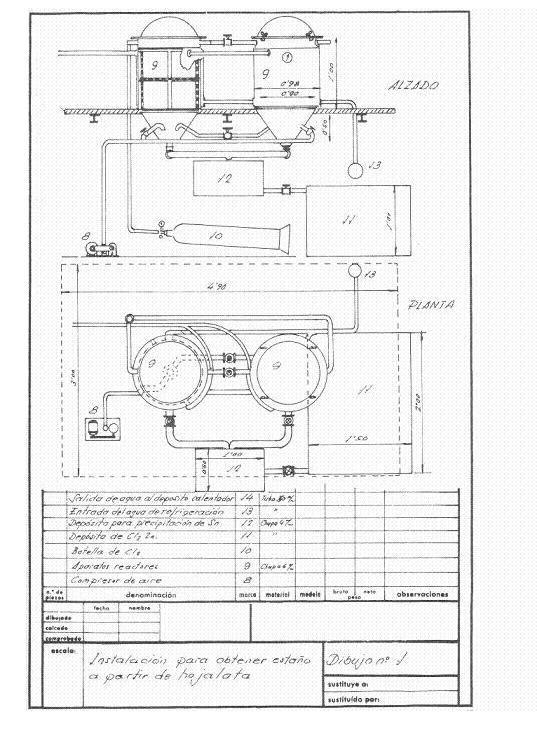
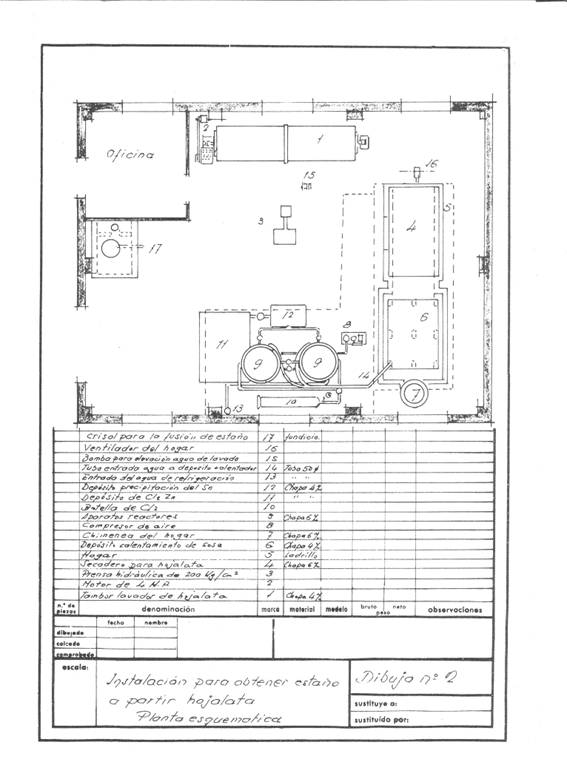
Figures 1 and 2 give an idea of the installation that was projected; it was thought in a frequency of recovery of 4 daily tons of cut working one shift and thought to work in campaigns.
Figure No. 1: Example 1 of installation of destaining
Figure nº2: Example 2 of installation of destaining
Upon receiving the cuttings in the installation, the first operation that was done was to wash them with approximately 2 cubic meters of a solution of 11-12 Be of sodium carbonate density; weighing 2160 kg and containing 1250 kg of crystalline soda – purity 98% -, treatment whose mission is to saponify the waste of fats that can contain the cuts and eliminate inks and varnishes.
Saponification is a chemical reaction between a fatty acid (or a saponifiable lipid , carrier of fatty acid residues) and a base or alkali , in which the salt of said acid and said base is obtained as the main product. These compounds have the peculiarity of being amphipathic , that is to say they have a polar part and another apolar (or non-polar), with which they can interact with substances of different properties. For example, soaps are salts of fatty acids and alkaline metals that are obtained through this process. The method of saponification in the industrial aspect consists of boiling the fat in large boilers, slowly adding caustic soda (NaOH), stirring the mixture continuously until it begins to become pasty.
This operation is carried out in a rotating drum of about 3.14 cubic meters of capacity and uses a washing surface of about 14.13 m2, built in 4 mm thick iron sheet and dragged by a 4 HP engine.
After the washing operation, the alkaline solution was decanted together with the soaps in a tank of about 3 m3 capacity, located above the drying oven of the tinplate to keep it warm and to be able to reuse it in new treatments until it is exhausted.
The grease-free, varnish and inks are rinsed with hot water, which is obtained generously in the cooling of the destaining reactors.
The clean cuts are pressed in prismatic packages of 400 x 170 x 200 mm, easily manageable, since they weigh about 50 kg, in a hydraulic press at about 200 kg / cm 2.
These packages are dried at atmospheric pressure at 200ºC for two hours, with an average consumption of 150 kg of coal of 7000 Kcal / kg. The drying chamber was a kind of shelf dryer – without much substance – built of “fortune”, where a medium fan injected the hot air to drag the water through the chimney: Everything was conveniently insulated with a magnesia layer of 85% , 60 mm thick and 280 kg in weight, and before sending it to the atmosphere it was given to yield excess calories to heat the solutions of the process, the 500 mm diameter chimney barely protruded from the roof.
The destatic reactors were two in series, in them and at their convenience dry air could be blown to pass the remnants of chlorine from the finished reactor to the other one, so that while one was untangling, the other one was extracting the stannic chloride and the recovered steel .
At the beginning of the day, the two reactors were loaded with pressed and dry tin packages. In the first one, dry chlorine was left at 2 atm. of pressure and the cooling water was allowed to flow, the chlorine gas expands on the free surface of the reactor and acts on the tin of tinplate; it continuously absorbs chlorine, while there is unreacted tin, and when all the tin has been transformed into stannic chloride the pressure remains constant and the attack is terminated. After a suitable period of time the apparatus is blown with dry air to draw the excess of chlorine to the canned reactor, which once closed begins to work, while all the stannic chloride formed by the finished reactor is removed from the bottom. blown, and when it is no longer dripping, the destained steel mass is removed, washed well with hot sodium carbonate solution, then rinsed with the hot water from the reactor and a sweet steel residue very advantageous for the steel mills is obtained. Working thus we have recovered 251 kg of stannic chloride and consumed 230-240 kg of chlorine.
This figure of recovered stannic chloride represents a recovery of 115 kg of metallic tin, that is, a recovery yield of 96%, with which the residual iron contains only 0.10-0.13% tin, which allows it to be sent to the steel mills No problem.
As the dissolution reaction is very exothermic, to achieve a good performance it is necessary to watch the refrigeration with great care, otherwise the risk of burning the can runs.
Liquid chlorine is commercially available in steel bottles similar to oxygen, acetylene, carbon dioxide, etc; they contain 50 kg of net weight at 6 atm and each kilogram of liquid chlorine supplies 334 liters of dry chlorine gas.
During the working time, we consumed 5 bottles per day to leach the 4 tons of clipping; we let expand the liquid chlorine of 6 to 2 atm, for which we used a commercial pressure reducer.
As metal tin was scarce at that time, we also prepared a reduction process for stannic chloride. To do this we plan to start up an electrolytic reduction without current consumption and with a minimum installation; It seems a contradiction, but after the relevant tests, the idea was not preposterous, we just had to develop an advantage of the difference in tension between zinc and tin. Studying the problem we reached a certainty: we saw that a sheet of zinc introduced in a solution of stannic chloride slightly acidulated with hydrochloric acid (pH = 5-6), carried out the following works:
Cl4Sn + 2 Zn = 2 Cl2Zn + Sn
Cl4Sn + Sn = 2 Cl2Zn
2 Cl2Sn + 2 Zn = 2 Cl2Zn + 2 Sn
What is translated into the consideration that all the zinc dissolves and that all the tin precipitates in spongy form of metallic character, that picks up, washes and refunds to obtain ingot.
According to the written reactions, 65 kg of zinc is required to recover all the tin of the stannic chloride formed. We used zinc sheets of 1 mm thickness that have a weight per square meter of 6.9 kg and therefore, we needed about 9.5 square meters of zinc sheet daily.
We made an installation of “fortune” for it. We built a container of 1000 x 600 x 500 mm and placed zinc sheets suspended on it from wooden frames. Once the precipitation was over we took the tin sponge with wooden pallets, we removed them and washed them well with hot water and melted them in an iron crucible easily at about 231.5º C with a coal consumption of 6-7% a pellet of 7000 Kcal / kg.
Once in a quiet fusion, the liquid was removed by means of green wood branches to deoxidize; the slag was removed and the purified liquid was introduced into ingot molds, which achieved a metal with a purity of 99.3 to 99.7% suitable for welding and manufacturing bronzes and other alloys.
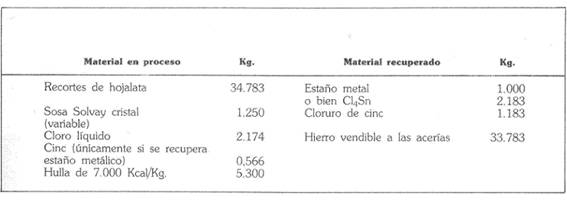
The table below gives a summary balance of the material of the operation, established for a production of one tonne of metal, equivalent to 2,183 kg of stannic chloride. “














Hi. My name is Ryan Patrick and I’m an engineer for CZero Energy in Santa Barbara, CA, USA. Our process involves producing carbon with some metal contamination. I am interested in setting up a call with someone who can speak to the process of metal leaching a substance like ours and the possible techno-economics of that. My phone number is (805) 298-6269.