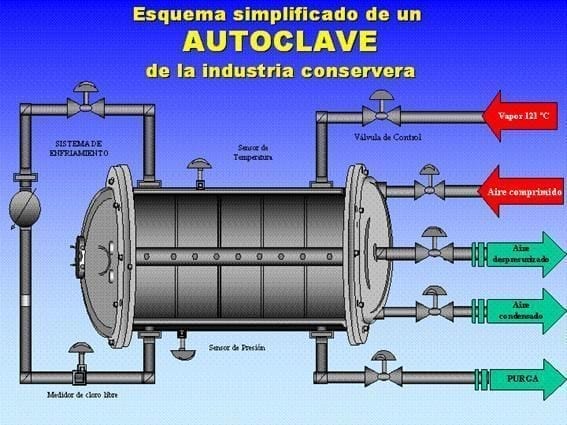
To control the pH and salts in the water used in the sterilization process, it is important to follow a series of recommendations:
- pH Control: Maintain the pH of the sterilization water between 7.00 and 8.00. Higher values may cause blackening of the tin by stannite formation and detachment of the varnish by an alkaline stripping effect. Use additive buffer products to obtain this ideal pH range.
- Salt Control: Ensure that chloride concentrations are less than 50 mg/l, alkalinity (expressed as CaCO3) is less than 350 mg/l, nitrates less than 25 mg/l, sulfates less than 150 mg/l and nitrites below 0.1 mg/l. If the water comes from a well or if the hardness index is high, it is recommended to apply an ultrafiltration treatment to reduce salinity levels.
- Automatic Flushing System: Install an automatic flushing system to avoid the concentration of saline residues due to water evaporation.
- Antioxidant Additives: Add additives with antioxidant character to the sterilization water to minimize corrosion processes.
- Boiler Water Quality Control: Keep the pH of the boiler feed above 8.5 and control conductivity to avoid corrosion. Use inhibitors such as sulfites and polymers to protect boilers.
- Specific Treatments for Boilers: In boilers, avoid corrosion with the addition of NaOH and control the pH with Na3PO4 to avoid excessive basification.
- Use of Low Acid Water: For cleaning autoclaves, use low acid cleaning products (pH below 6) and clean with distilled or demineralized water.
By following these recommendations, you can effectively control the pH and salts in the water and reduce corrosion problems in metal containers during sterilization.