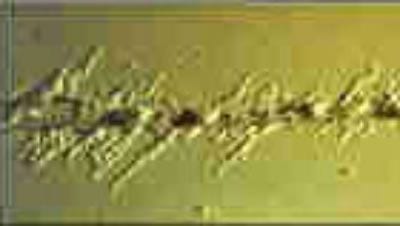
One of the corrosion problems that most concerns the container manufacturer and the canner is the appearance of a type of corrosion that appears on the container and lids in the form of filaments that do not follow a defined shape but usually have a predetermined direction, following the direction of metal lamination. It is a differential crevice or crevice corrosion and occurs in thin organic coated thin metals. It is known as filiform or “worm” corrosion and occurs in steel and aluminum containers, stored for a long time (several months) whether or not they have been processed.
Filiform corrosion on the closure of a processed container after prolonged storage. | Filiform corrosion under varnish on the flange of an unprocessed stored container |


Filiform corrosion usually starts in scratches or other defects in the coating and spreads laterally as narrow filaments 0.05 to 3 mm wide under the coating. Penetration into the metal substrate is generally low, only superficial.

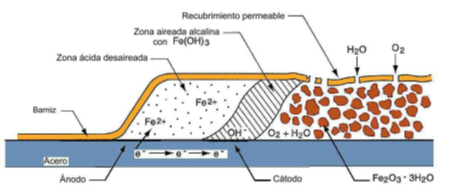
The strands consist of an active corrosion head, followed by an inactive tail filled with corrosion products. In steel, the head of the filament is usually blue-green, or gray, and the tail is an oxidized red, indicating that the head is de-aerated while the tail is aerated.
Oxygen is consumed by active corrosion in the head and is accompanied by hydrolysis and acidification at pH 1 to 4. Filiform corrosion is enhanced by atmospheric constituents such as soluble chlorides, sulfates, sulfides or carbon dioxide, which aid acidification during differential aeration. The precipitation of red Fe(OH)3 oxide occurs when Fe+2 from the head contacts the aerated conditions at the tail of the filament. Essential water and oxygen migrate to the corrosive strand head through porosity or micro cracks in the coating over the strand tail. Fe (OH)3 decomposes into Fe2O3- H2O as the filament advances. The process is shown schematically in the following Figure:(Taken from Jones, D.A. Principles and Prevention of Corrosion):
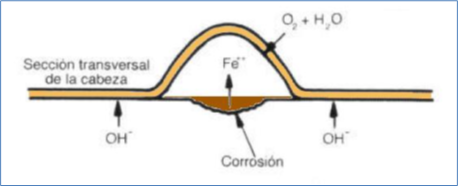
Seen in profile, the head of the corrosion filament has the following appearance: (Taken from OTERO HUERTA, E. Corrosion and degradation of materials).
Sealing the tail against water and oxygen transport deactivates a strand. One propagating filament cannot cross the path of the other, presumably because the tail of the former provides a source of oxygen that interrupts the propagation of the filament cell. The permeability of the coating and its composition have little or no effect on the initiation and growth characteristics of filiform corrosion. The growth of the growing filaments is not affected by the type or thickness of the coating.
The only sure way to avoid filiform corrosion on steel, aluminum, or magnesium is to reduce the relative humidity nominally below 60%, thereby dehydrating the filament cell.
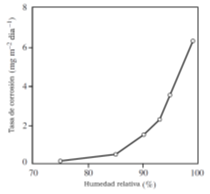
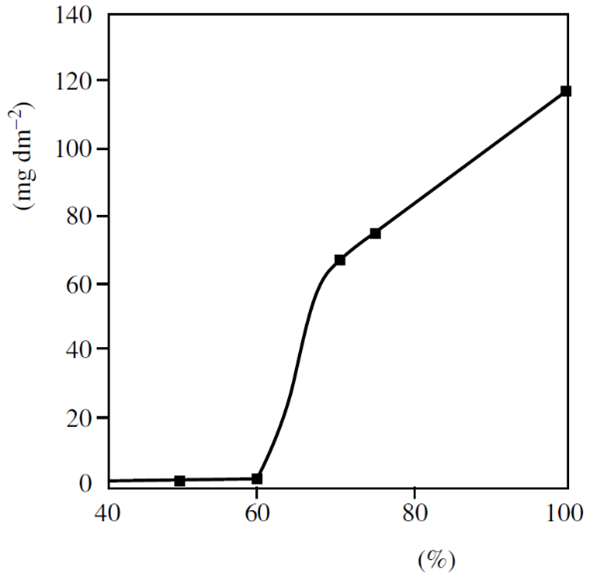
Improved coating quality, multiple coatings, and inhibition of coatings and primers may retard, but do not totally prevent filiform corrosion in operating equipment where the atmosphere cannot be controlled. Corrosion resistant substrates of stainless steel, titanium, or copper do not exhibit filiform corrosion.














0 Comments