It is mainly interesting to examine the behavior of tinplate as a raw material for the manufacture of containers, and specifically from the point of view of corrosion.
Atmospheric agents attack tinplate according to ambient humidity, average temperature, surface treatment, changes in temperature, thickness and quality of the tin coating, etc. Undoubtedly, there are other elements that can come into contact with the outside of a container, and cause a deterioration of tinplate, but in this case we intend to briefly describe what happens, or can occur with the inside of the container. And within it, only the alterations of chemical or electrochemical type, leaving aside the microbiological type, for its tremendous extension and complexity. These alterations are designated as corrosion although they can really be a more complex phenomenon.
The effects of this corrosion, can make the contents of the container inedible, lose their essential characteristics, that the package alter the normal appearance or that is perforated. In any case, this implies that the continent-content set is unusable; the time elapsed between the filling of the container until this phenomenon occurs, supposes the “useful life” of the same.
The products that are packaged are of a great variety, there are very acidic, very aggressive towards metals, etc. However, tinplate remains free of corrosion for long periods. One reason for the slow dissolution of tin, is its relatively high electrical potential in relation to hydrogen, which slows down the reaction in which hydrogen is released on the surface of that metal, the latter passing to the liquid medium. in oxidizing media, hydrogen atoms can react with oxygen to form water, and corrosion accelerates; here is one of the fundamental reasons for preheating preserves and thus eliminate gaseous or dissolved air and also the need to get a good vacuum.
In spite of everything, the chemical properties of tin are insufficient to satisfactorily explain its good behavior. The tin is distributed in a very thin layer and does not act as an insulating layer: there are pores that leave the steel exposed. This peculiarity makes them act like tiny electric batteries; two electrodes submerged in a conductive liquid and electrically connected to each other. Depending on the content, tin may be anodic or cathodic relative to steel.
In the first case, which is the most common, the tin is slowly dissolving, that is, it protects the steel, “sacrificing itself”. While tin is present in metallic form and making direct electrical contact with the iron, there will be no perforation of the container. If the tin acts as a cathode, the anodic corrosion concentrates in the pores of bare iron and there may be rapid perforations.
Normally, there must be a release of gaseous hydrogen at the cathode, a gas that is replacing the vacuum of the head space, which can create a positive pressure in it. This formation of internal pressure may also be due to some microbiological process, without forgetting that some types of bacteriological alteration do not generate gases. When there has been an increase in sufficient internal pressure, the bottom or the lid or both, must undergo a deformation in its center, indicating to the consumer that something abnormal has occurred inside the can, whose content may be in bad conditions for its consumption. If there has been alteration without gas production, there will be no outside signal to indicate it.
When it comes to sterilized preserves, during the process a certain internal pressure is generated in the container, which tends to bulge this. This pressure is caused by the increase in volume of the product as it expands due to the action of heat. If there is an air chamber (head space), it decreases, increasing the pressure – above the atmospheric pressure – and returning to its original negative value (vacuum) when cooling. This momentary change in pressure produces a transient deformation in the caps, which subsequently disappears. If originally there is no vacuum or there is no head space, the internal pressure is so great that the deformation in the caps is permanent and irreversible, producing a preserves with all the appearance of suffering a serious alteration. From all this it follows the need for the profiles of lids and bottoms are deformable and elastic within limits, so as to allow adsorb reasonable variations of pressure during the process but not the resulting, – more accentuated – a fermentation or internal corrosion.
The internal corrosion of a container, – that is, the dissolution of tin in the contents of the can – is not harmful to the consumer, it only generates variations in taste, smell and presentation in the product. Therefore, a container domed by generation of hydrogen by corrosion, the only thing that indicates is that it is an old canned, which has already exceeded its limit of useful life.
There is another common type of attack of the contents of a container to the container. It is not corrosion. Are the reactions with tinplate of the sulfur compounds existing in the product or released during sterilization. The reaction can be with tin or iron and produces brown, gray or black spots of different appearance depending on the intensity of the reaction, the type of tinplate, etc. In the worst cases, the product of the reaction can be mixed with the governing liquid or adhere to the content, and although they are not harmful to health, they give a very bad presentation. The solution to this problem is the use of sanitary varnishes, nowadays very widespread.
From the above it can be inferred that by the action of corrosion, the storage life of a can is dependent on many factors, but other things being equal, it varies with the thickness of the tin coating inside the container.
As already indicated, the use of varnishes is the most usual technique, at least on top and bottom. When the product is not aggressive, varnishes are used to improve presentation. The use of these can reduce the tin coating, which largely compensates the cost of the varnish, depending on the decrease in tin and the amount of varnish, which is a function of quantities and qualities.
In the case of corrosive products, it is normal to interiorly varnish the entire container. This requires a good quality of application of the varnish and even then always presupposes a certain risk since the surface of exposed tin is very small – pores in the varnish -. In these pores if the tin acts cathodically, it protects the iron for a certain time, but not very long since there is little tin “available” going soon to the process of perforation of the wall of the container. If the tin acts anodically, the drilling process starts immediately. If the risk is high, it is necessary to resort to double layer of total varnish and even to total internal rebarnizado after the conformation of the container.





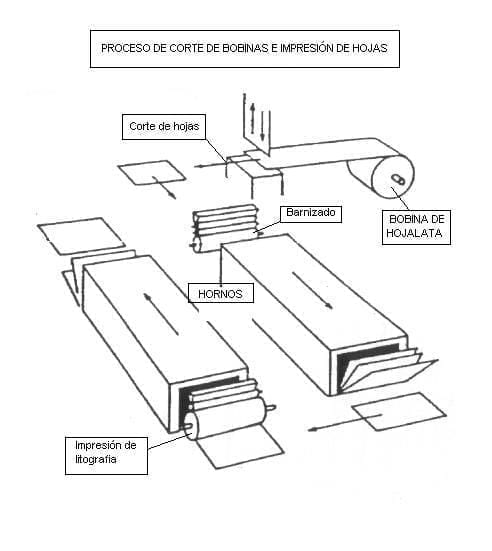








0 Comments