STUDY OF THE LANGELIER INDEX: INFLUENCE OF DIFFERENT PARAMETERS ON WATER AGGRESSIVENESS
As we already know, the problems of external corrosion of containers is one of the main problems that appears in the packaging industry, and which causes most of the complaints from canners. In many cases, the treatment water is the main cause of corrosion. Let’s take a look at one of the water testing methods that can help us control this problem.
The Langelier saturation index (LSI) is an equilibrium model derived from the theoretical concept of saturation and provides an indicator of the degree of saturation of water with respect to calcium carbonate. It is one of the indices used as a reference to assess the corrosiveness of water.
The LSI is probably the most widely used indicator of cold water potential scale. It is purely an equilibrium index and deals only with the thermodynamic driving force for the scale of formation and growth of calcium carbonate.
To calculate the LSI, it is necessary to know the alkalinity (mg/l as CaCO3), calcium hardness (mg/l Ca2+ as CaCO3), total dissolved solids (mg/l TDS), pH and current water temperature(oC). If TDS is unknown, but conductivity is known, can estimate TDS in mg/L using a conversion factor widely used in the industry.
LSI is defined as: LSI = pH – pHs
| Where: pH is the measure of the pH of water pHs is the pH at saturation in calcite or calcium carbonate, and is defined as: |
pHs = (9.3 + A + B) – (C + D)
| A = (Log10 [TDS] – 1) / 10 B = -13.12 x Log10 (oC+ 273) + 34.55 C = Log10 [Ca2+ as CaCO3] – 0.4 D = Log10 [alkalinity as CaCO3] Therefore, together with the pH of the water we have five factors that influence the Langelier Index. |
We will see how the variability of each of them affects the Langelier index. To do this, we start with water parameters that can be considered normal in an industrial water, and by setting four of them, we vary the other element within tolerable industrial parameters. We establish, therefore, the chemical characteristics of this water, and the range of each one of the parameters, would be:
pH= 7.6 and we will estimate pH changes from 6.0 to 8.5.
Alkalinity =90 mg/l (as CaCO3) and we estimate a range between 25 and 140 mg/l.
Hardness= 300 ppm (as CaCO3) and we estimate a range of 20 to 400 ppm.
TDS = 800 ppm and we estimate a range between 50 and 1200 ppm.
Tº: The LSI is calculated for two temperature values 25 and 85ºC, except for the evaluation of the temperature change, which will be done from 10 to 120ºC.
For a variation in pH between 6 and 8.5 we obtain the following results:
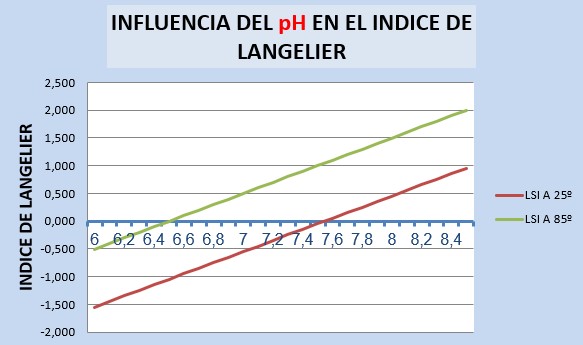
The LSI varies over a range of approximately 2.5 points, from -1.55 to 0.95 for cold water and from -0.5 to 2.0 for water at 85°C.
For changes in alkalinity between 25 and 140 mg/l:
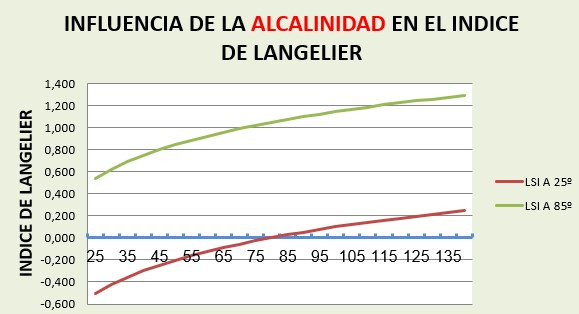
The LSI changes span a range of approximately 0.75 points varying from -0.5 to 0.245 for cold water and from 0.55 to 1.29 for 85º water.
For changes in total dissolved solids (TDS) from 50 to 1200 ppm:
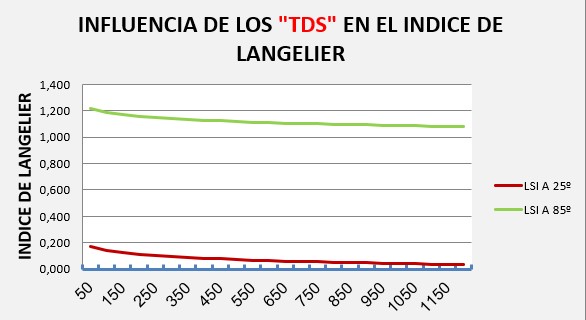
The LSI varies from 0.17 to 0.035 for cold water and from 1.22 to 1.08 for water at 85º, i.e. it has a variation range of approximately 0.15 units.
For changes in water hardness from 20 to 400 ppm, we would have:
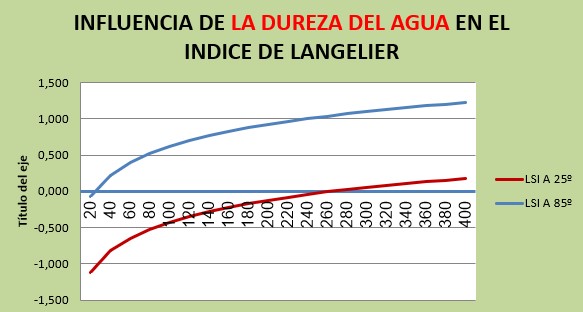
The LSI varies in a range of approximately 1.3 points, going from -1.12 to 0.18 for cold water and from -0.08 to 1.22 for water at 85ºC.
Finally, let us look at the influence of temperature on LSI changes. Although we know that we can have little influence on this, being process temperatures, it is also convenient to know how it affects the Langelier Index in the water:
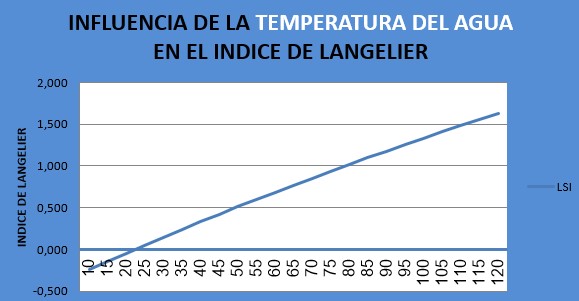
We see that the temperature from 10 to 120ºC varies the Langelier index in a range of almost 2 points, going from -0.24 to 1.63.
In view of all these factors, it is easy to understand how we can vary the Langelier Index, and above all, that those elements that are more easily manipulated in water, such as pH, hardness and temperature, are precisely those that most affect the index, within the established ranges of variability.
Similarly, we see that alkalinity and TDS do not have an important influence on LSI, although TDS, for example, does have other precipitating influences on other salts and above all on the increase in conductivity, which would give us another type of corrosion (galvanic).
We know that an LSI below -0.5 indicates that the water is corrosive, the lower the LSI, the more corrosive the water is.
On the other hand, a positive Langelier index (>0.5) indicates that the water is precipitating, i.e. it deposits carbonates, the higher the LSI, the more it would stain the containers and could lead to corrosion during storage.








0 Comments